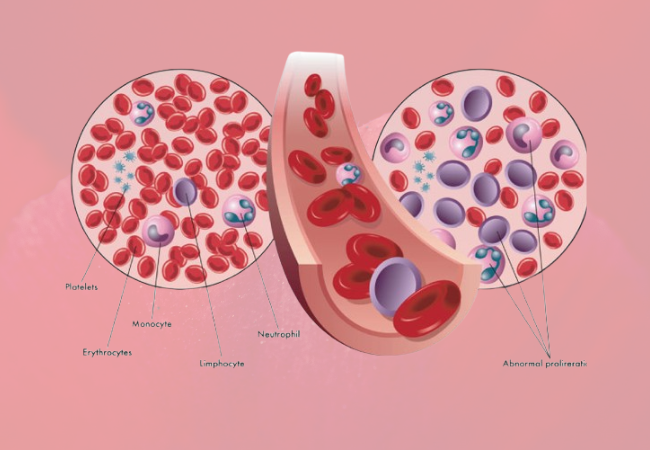
The U.S. Food and Drug Administration (FDA) has granted its approval for a new therapy aimed at treating myelodysplastic syndromes (MDS), a rare category of blood cancers characterized by mutations in bone marrow progenitor cells, leading to insufficient healthy blood cell production. This groundbreaking development brings hope to the 60,000 to 170,000 individuals living with MDS in the United States and approximately 87,000 new cases diagnosed worldwide each year, with about 3.6 percent of MDS patients harboring an isocitrate dehydrogenase-1 (IDH1) mutation.
The FDA’s approval includes the use of Tibsovo (ivosidenib) for the treatment of adult patients with relapsed or refractory MDS who carry the IDH1 mutation, as identified through an FDA-approved test. This approval marks a significant milestone as it is the first targeted therapy specifically designed for this indication. Additionally, the agency has approved the Abbott RealTime IDH1 Assay as a companion diagnostic to help select suitable MDS patients with the IDH1 mutation for treatment with Tibsovo.
Richard Pazdur, M.D., director of the FDA’s Oncology Center of Excellence, emphasized the importance of this approval, stating, “Today’s approval represents an important treatment advancement for rare blood cancers, and more specifically, patients with relapsed or refractory MDS who have an IDH1 mutation.” He also emphasized the FDA’s commitment to advancing the development of safe and effective therapies for patients with rare cancers through their Rare Cancers Program.
Tibsovo was previously approved for various conditions, including newly diagnosed acute myeloid leukemia (AML), relapsed or refractory AML, and locally advanced or metastatic cholangiocarcinoma. The Abbott RealTime IDH1 Assay was also approved as a companion diagnostic for AML patients with the IDH1 mutation. The new indication’s effectiveness was evaluated in a study involving 18 adult patients with relapsed or refractory MDS and the IDH1 mutation, with encouraging results.
While ivosidenib has shown promise, it comes with potential side effects, such as diarrhea, nausea, joint pain, and more. It can also lead to QTc prolongation, causing abnormal heart rhythms. A boxed warning for differentiation syndrome has been included in the prescribing information for Tibsovo, as it can be fatal if not treated promptly. This syndrome is marked by various symptoms, including fever, difficulty breathing, and inflammation in the lungs.
Tibsovo’s approval came with Priority Review designation, which aims to expedite drug applications for serious conditions. It also received FDA Breakthrough Therapy and Orphan Drug designations to encourage and support the development of therapies for rare diseases.
This FDA approval for Tibsovo offers new hope for those battling the challenges of myelodysplastic syndromes, underscoring the significance of advancing targeted therapies for rare and life-threatening conditions.
Reference:
https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-myelodysplastic-syndromes?utm_medium=email&utm_source=govdelivery