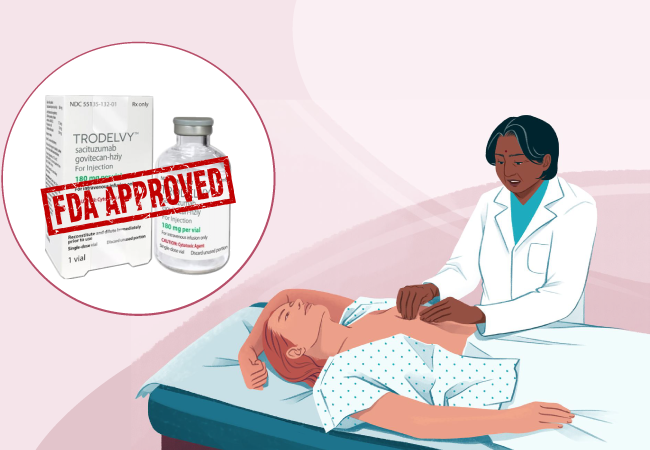
On February 2023, the FDA approved the medicine Trodelvy (sacituzumab govitecan-hziy) for the treatment of unresectable locally advanced or metastatic hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the metastatic setting.
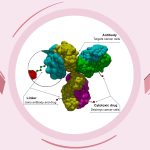
Trodelvy comes in the form of 180 mg lyophilized powder in single-dose vials for reconstitution. Trodelvy 180 mg is a Trop-2-directed antibody and topoisomerase inhibitor conjugate composed of the following three components:
- The humanized monoclonal antibody, hRS7 IgG1κ (also called sacituzumab), which binds to Trop-2 (the trophoblast cell-surface antigen-2);
- The drug SN-38, a topoisomerase inhibitor;
- A hydrolysable linker (called CL2A), which links the humanized monoclonal antibody to SN-38.
The primary efficacy outcome measure was progression-free survival determined by an anonymous independent central review per RECIST v1.1. A key secondary efficacy outcome measure was overall survival. Median progression-free survival was 5.5 months in the sacituzumab govitecan-hziy arm and 4 months in the single-agent chemotherapy arm. Median overall survival was 14.4 months for those receiving sacituzumab govitecan-hziy and 11.2 months for those receiving single-agent chemotherapy.
The common side effects of sacituzumab govitecan-hziy are decreased leukocyte count, decreased neutrophil count, decreased hemoglobin, decreased lymphocyte count, diarrhea, fatigue, nausea, alopecia, increased glucose, constipation, and decreased albumin.
Reference: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-trodelvy-sacituzumab-govitecan-hziy-hr-positive-breast-cancer#:~:text=On%20February%203%2C%202023%2C%20the,at%20least%20two%20additional%20systemic