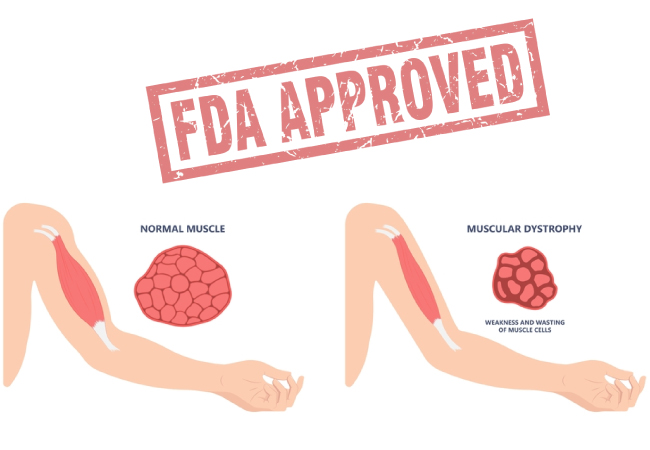
The U.S. Food and Drug Administration (FDA) has recently approved Duvyzat (givinostat), an FDA-approved nonsteroidal oral treatment for Duchenne Muscular Dystrophy (DMD) in ambulatory patients aged 6 years and older. This regulatory milestone adds a new therapeutic option to the current treatment landscape for DMD.
Duvyzat is a histone deacetylase (HDAC) inhibitor that acts through an epigenetic mechanism, targeting multiple pathways involved in inflammation and muscle degeneration. Unlike some mutation-specific therapies, Duvyzat is not mutation-dependent and may be applicable across a broader population of patients with DMD.
Duchenne Muscular Dystrophy is a rare, X-linked genetic disorder that leads to progressive muscle degeneration and weakness. It is caused by mutations in the dystrophin gene, resulting in reduced or absent dystrophin protein, essential for muscle cell stability. DMD primarily affects boys and typically manifests between the ages of 2 and 5, with a gradual loss of motor function over time.
Existing Treatment Limitations:
Corticosteroids have been the mainstay treatment for DMD, helping to slow disease progression. However, they are often associated with adverse effects such as weight gain, osteoporosis, growth suppression, and mood changes. This has led to increasing interest in alternative therapeutic approaches with potentially improved tolerability and different mechanisms of action.
How Duvyzat Works?
Duvyzat (givinostat) is a nonsteroidal oral HDAC inhibitor. It is designed to modulate gene expression by epigenetic regulation, reducing inflammation and fibrosis while preserving muscle tissue. Its mechanism is not tied to specific dystrophin gene mutations, allowing broader patient eligibility.
The approval was supported by results from a Phase 3 randomized, double-blind, placebo-controlled study conducted in ambulatory boys with DMD. All patients in the study continued their standard steroid treatment during the trial.
Key Clinical Study Insights:
The 18-month study assessed physical function using multiple measures:
Primary Endpoint:
- Mean change in the four-stair climb test from baseline to Month 18.
- Duvyzat group: 1.25-second increase
- Placebo group: 3.03-second increase
- Suggesting a slower decline in stair climbing ability with Duvyzat.
Secondary Endpoint:
- Change in scores on the North Star Ambulatory Assessment (NSAA).
- Patients treated with Duvyzat showed less worsening in their NSAA score compared to placebo.
Safety Profile of Duvyzat:
The most commonly reported adverse reactions in clinical trials included:
- Fever
- Diarrhea
- Abdominal pain
- Reduction in platelets (which may increase
- bleeding risk)
- Nausea
- Vomiting
- Elevated triglycerides
Patients receiving Duvyzat should be monitored for these effects during treatment.
Dosage and Administration:
Duvyzat should be administered orally, twice daily with food. The dosage is based on the patient’s body weight and should be followed as per the prescribing physician’s recommendations.
Conclusion: The FDA approval of Duvyzat (givinostat) offers a new treatment avenue for patients with Duchenne Muscular Dystrophy aged six years and older. As research into rare neuromuscular diseases advances, innovations like Duvyzat reflect ongoing efforts to expand therapeutic options for individuals with limited choices. Continued collaboration between researchers, regulators, physicians, and patient communities remains essential in bringing new solutions to those affected by rare disorders like DMD.
References: FDA Official Press Release
What Does This Mean for the DMD Community?
For individuals living with DMD and their caregivers, the availability of a nonsteroidal oral therapy like Duvyzat represents an important addition to treatment options. This approval provides a new strategy for managing functional decline and enables further personalization of care, particularly for patients who may not respond well to or tolerate steroids.