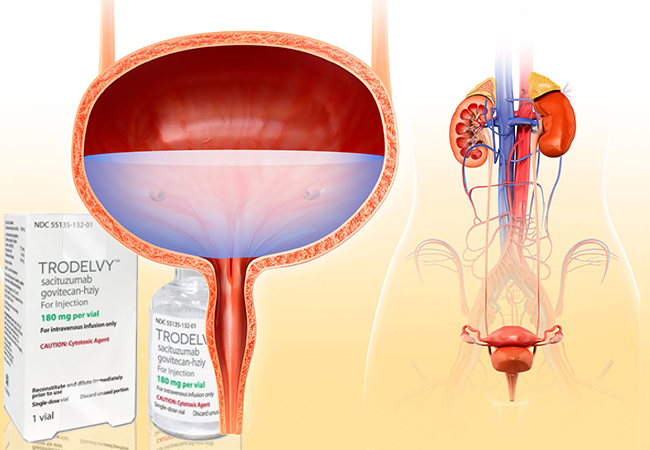
Trodelvy (sacituzumab govitecan-hziy) is a unique chemotherapy drug that specifically targets and attacks certain cancer cells. Designed to treat cancers that don’t respond well to other treatments, Trodelvy brings hope to patients, particularly those dealing with challenging cancers like urothelial carcinoma (UC), a type of bladder cancer. In India, where cancer rates are steadily rising, Trodelvy offers an alternative for patients who have few treatment options left.
Which Types of Cancer is Trodelvy Approved to Treat?
Trodelvy has been approved to treat:
- Triple-Negative Breast Cancer (TNBC): For patients who have tried at least two prior treatments. TNBC doesn’t have hormone receptors, so traditional therapies aren’t effective.
- HR+/HER2- Breast Cancer: Hormone receptor-positive, HER2-negative breast cancers can be treated with Trodelvy after hormone therapy, and at least two other therapies for metastatic cancer have been attempted.
- Advanced Urothelial Cancer: Trodelvy is used for patients whose cancer hasn’t responded to platinum-based chemotherapy and immunotherapy.
Trodelvy’s Effectiveness in Advanced Urothelial Cancer:
For people with advanced urothelial cancer in India, Trodelvy represents a major advancement, especially for those who have exhausted conventional treatments. Clinical trials like TROPHY-U-01 have demonstrated that Trodelvy has promising results in controlling urothelial cancer:
- Progression-Free Survival (PFS): Patients achieved a median PFS of 5.4 months.
- Overall Survival (OS): The median OS time was 10.9 months, meaning patients lived for nearly 11 months after starting Trodelvy.
- Response Rate: Around 27% of patients in the TROPHY-U-01 trial had a positive response to treatment, with either significant tumor shrinkage or stabilization of their disease.
In many cases of advanced urothelial cancer, typical survival averages around 7–8 months. With Trodelvy, survival times are extended, giving patients and families more hope. Without it, only about 10% of patients would see any positive response, underscoring the effectiveness of this innovative therapy.
Trodelvy’s Role in Tumor Reduction and Disease Stability:
One of Trodelvy’s notable effects is its ability to reduce tumor size:
- In urothelial cancer trials, 27% of patients saw tumor reduction, with 5% experiencing complete disappearance of tumors and 22% seeing tumors shrink by at least 30%.
- Stability was another key benefit, with Trodelvy helping to halt the growth of tumors in several patients, even if tumors didn’t disappear completely. This stability allows patients to experience fewer symptoms and gain valuable time.
Recent Advances: FDA Approvals and Indian Access:
In the evolving landscape of cancer treatments, Trodelvy stands out for its focused mechanism that effectively slows down or halts the growth of aggressive cancers. Indian healthcare providers now have improved access to Trodelvy and other recent approvals like Pembrolizumab and Atezolizumab, both of which are effective in treating advanced urothelial cancer. As these drugs become more available in India, patients with limited options have new hope for prolonged, meaningful survival.
Why Trodelvy Offers Unique Benefits:
Trodelvy offers several advantages that make it a valuable option in advanced cancer cases:
Targeted Treatment: Unlike conventional chemotherapy, which affects all rapidly dividing cells, Trodelvy’s targeted design allows it to zero in on cancer cells specifically, minimizing the impact on healthy cells.
Improved Survival Outcomes: Studies show Trodelvy can extend both overall survival and progression-free survival, particularly for patients with advanced urothelial cancer.
Quality of Life (QoL): With fewer side effects compared to broader chemotherapy, Trodelvy can improve quality of life, allowing patients to feel better as they undergo treatment.
Frequently Asked Questions (FAQs):
What is the primary benefit of Trodelvy for urothelial cancer patients?
Trodelvy directly targets cancer cells, reducing tumor size and slowing progression. This can lead to longer survival and a reduction in symptoms.
Who should consider Trodelvy for urothelial cancer treatment?
Patients with urothelial cancer who have tried platinum-based chemotherapy and immunotherapy without success may benefit from Trodelvy. Consult your doctor to see if it’s a suitable option.
Are there side effects with Trodelvy?
Common side effects include nausea, fatigue, and low white blood cell count. However, the side effects vary, and your healthcare team can provide support to manage them effectively.
How can I buy Trodelvy in India?
To buy Trodelvy in India, contact authorized suppliers like the Indian Pharma Network (IPN) for verified access. Reach out via Call/WhatsApp: +91 9310090915 or TOLL-FREE: 1800-889-1064 for reliable support and guidance.
How does Trodelvy compare to traditional chemotherapy?
Trodelvy is more targeted, reducing cancer cells specifically, which often leads to fewer side effects and better outcomes than traditional chemotherapy.
How long can Trodelvy help extend survival?
Studies indicate that Trodelvy can extend overall survival to around 10.9 months for urothelial cancer patients, though results can vary depending on individual factors.
Conclusion:
As a promising option for advanced urothelial cancer in India, Trodelvy is helping to reshape treatment approaches, offering better outcomes where few options remain. With its targeted mechanism and improved survival rates (SR), Trodelvy brings a new level of hope to patients and families. For further information or access, Contact Indian Pharma Network (IPN) via Call/WhatsApp: +91 9310090915 or TOLL-FREE: 1800-889-1064.