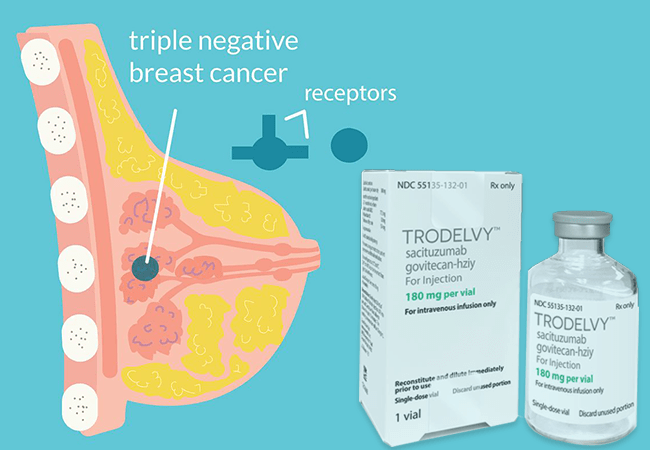
Trodelvy (sacituzumab govitecan-hziy) is an innovative antibody-drug conjugate (ADC) and has emerged as a game-changer in treating metastatic triple-negative breast cancer (mTNBC), as well as other challenging forms of metastatic breast cancer. Manufactured by Gilead Sciences, it is available in 180 mg and 200 mg single-dose vials for reconstitution. Trodelvy’s unique mechanism of action and expanding uses bring new hope to patients facing advanced cancer.
Mechanism of Action:
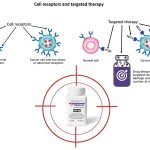
Trodelvy works by targeting a specific protein, Trop-2, which is commonly overexpressed on the surface of cancer cells in several types of breast cancer. The drug is composed of three essential parts:
- Sacituzumab – a monoclonal antibody that binds directly to the Trop-2 protein on cancer cells.
- SN-38 – a powerful chemotherapy agent that inhibits topoisomerase-I, essential for DNA replication in cancer cells.
- Linker – a connector that attaches SN-38 to the antibody.
When Trodelvy binds to Trop-2, it is absorbed into the cancer cell, and the linker is cleaved, releasing the SN-38 chemotherapy directly inside the tumor. This method helps selectively target cancer cells while limiting damage to healthy cells, enhancing treatment effectiveness.
Approved Indications:
Trodelvy is FDA-approved and offers an important treatment option for patients who have exhausted other therapies. Its indications include:
- HR+/HER2- Metastatic Breast Cancer: After prior hormone therapy and two chemotherapy regimens, Trodelvy helps patients with HR-positive, HER2-low metastatic breast cancer.
- Metastatic Triple-Negative Breast Cancer (mTNBC): For patients with mTNBC who have tried at least two prior therapies, Trodelvy is a preferred choice, especially for its superior clinical results.
- Advanced Bladder Cancer: Trodelvy has also gained approval to treat patients with advanced bladder cancer who have undergone prior treatments.
Clinical Efficacy:
Metastatic Triple-Negative Breast Cancer (mTNBC): In the pivotal ASCENT trial, Trodelvy showed remarkable efficacy for mTNBC patients, demonstrating a median progression-free survival (PFS) of 5.6 months compared to 1.7 months with chemotherapy. Additionally, the median overall survival (OS) was 12.1 months for Trodelvy compared to 6.7 months with chemotherapy, offering a substantial improvement in outcomes.
HR+/HER2- Metastatic Breast Cancer: Trodelvy’s approval for HR-positive, HER2-low metastatic breast cancer was based on the TROPiCS-02 trial, which showed that Trodelvy improved PFS and OS in patients with this cancer subtype. Median PFS was 5.5 months for Trodelvy, compared to 4.0 months for chemotherapy, while OS extended to 14.4 months versus 11.2 months for chemotherapy.
Advanced Bladder Cancer: In treating advanced bladder cancer, Trodelvy has demonstrated similar efficacy, improving patient survival and reducing cancer progression. Its approval in this area opens doors for individuals with limited options in bladder cancer management.
Ongoing Clinical Trials:
Trodelvy is currently being explored in several ongoing trials, aiming to widen its scope of use and explore its effectiveness in combination with other treatments:
- ASCENT-03 Trial: Evaluates Trodelvy as a first-line treatment for mTNBC.
- TROPiCS-04 Trial: Studies Trodelvy in HR-positive, HER2-low metastatic breast cancer patients who have tried CDK4/6 inhibitors.
- SEASTAR Trial: Examines Trodelvy in combination with other targeted therapies for mTNBC.
- TALENT Trial: Explores Trodelvy’s utility in patients with advanced solid tumors, including non-small cell lung cancer and urothelial carcinoma.
Availability and Access:
Trodelvy is accessible across Asia and the Middle East, including countries like UAE, Kuwait, Iraq, Oman, Qatar, Saudi Arabia, and Bahrain, through the Indian Pharma Network, which ensures a reasonable Trodelvy price. Trodelvy injection can also be made available in Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan, and Sri Lanka.
Patient Feedback and Support:
Patient organizations, like Rethink Breast Cancer and the Canadian Breast Cancer Network, have gathered feedback from patients undergoing Trodelvy treatment. Many patients reported positive experiences, including significant relief from symptoms and improvements in quality of life. However, there is still a need for broader accessibility and support due to the high costs of advanced cancer treatments.
Conclusion:
Trodelvy is redefining the landscape of metastatic breast cancer treatment, offering life-extending benefits and enhanced quality of life for patients with limited options. Through the Indian Pharma Network (IPN), this innovative therapy is available globally, giving hope to those battling complex cancer types. For further information on Trodelvy and its availability, contact the IPN via Call/WhatsApp: +91 9310090915.
References:
https://www.ema.europa.eu/en/medicines/human/EPAR/trodelvy
https://everyone.org/blog/trodelvy-information
How successful is Trodelvy?
Trodelvy has shown significant success in clinical trials. Patients treated with Trodelvy were three times more likely to be progression-free at one year compared to those receiving standard chemotherapy (21% versus 7%). Additionally, Trodelvy 180 mg and 200 mg demonstrated notable improvements in secondary endpoints, including a higher objective response rate and extended time to deterioration (TTD).
How long does Trodelvy extend life?
Trodelvy has been shown to extend life by approximately 29% compared to previous treatments. This is measured as the median overall survival (OS), indicating that half of the patients receiving Trodelvy lived about 29% longer after starting treatment compared to those on alternative therapies.
What is the target of Trodelvy?
Trodelvy specifically targets cells expressing the Trop-2 protein. Trop-2 is a cell surface protein that Trodelvy binds to in order to deliver its therapeutic effects.
Is Trodelvy expensive?
The cost of Trodelvy injection through the Indian Pharma Network is reasonable making it easier to incorporate in the treatment.
Does Trodelvy shrink tumors?
Yes, Trodelvy has been effective in shrinking tumors in clinical studies. About one-third (33%) of patients experienced tumor shrinkage, and more than half (55%) had no cancer progression for at least six months while on Trodelvy.
What are the primary uses of Trodelvy?
Trodelvy is approved for HR+/HER2- metastatic breast cancer, metastatic triple-negative breast cancer (mTNBC), and advanced bladder cancer. It serves patients who have previously received other treatments but need more effective options.
How does Trodelvy work to treat cancer?
Trodelvy targets Trop-2 protein on cancer cells. Once attached, it delivers SN-38, a potent chemotherapy, directly to the cancer cells, minimizing damage to healthy tissues and improving treatment precision.
What are the potential side effects of Trodelvy?
Common side effects of Trodelvy include fatigue, nausea, hair loss, and diarrhea. Although generally well-tolerated, some patients may experience severe side effects, and regular monitoring is essential during treatment.
How effective is Trodelvy in shrinking tumors?
In clinical trials, approximately 33% of mTNBC patients experienced tumor shrinkage, with 55% having no progression for at least six months, demonstrating Trodelvy’s high efficacy in controlling cancer.
Where can I buy Trodelvy, and is it affordable?
Trodelvy can be made available in India and other parts of Asia through the Indian Pharma Network, which provides it at a reasonable cost, making it more accessible for those needing advanced cancer treatment options.
How can I buy Trodelvy in Middle Eastern countries?
Buy Trodelvy in India and Middle Eastern countries, including Kuwait, Iraq, Oman, Qatar, and Bahrain, through the Indian Pharma Network (IPN), which ensures affordable options. For details, contact via Call/WhatsApp: +91 9310090915.