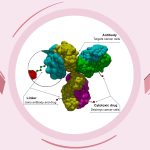
Trodelvy (sacituzumab govitecan-hziy) is the first FDA-approved antibody-drug conjugate (ADC) for treating advanced triple-negative breast cancer (TNBC) in adults. It is indicated for patients who have previously undergone at least two treatments for metastatic disease.
Currently, this therapeutic drug is also approved for pre-treated HR+/HER2- metastatic breast cancer and for second-line treatment of metastatic TNBC in patients who have received endocrine-based therapy and at least two additional systemic treatments.
Additionally, Trodelvy is used to treat locally advanced or metastatic urothelial cancer (mUC) in patients who have previously been treated with platinum-based chemotherapy and a PD-1 or PD-L1 inhibitor.
Developed by Immunomedics, Trodelvy became part of Gilead Sciences’ portfolio after Gilead acquired Immunomedics for $21 billion in October 2020. Trodelvy is administered intravenously and is available as a 180 mg lyophilized powder, which is off-white to yellow in color, in single-dose vials for reconstitution.
Regulatory approvals for Trodelvy:
In May 2018, Immunomedics submitted a biologics license application (BLA) for Trodelvy to the US Food and Drug Administration (FDA). Initially rejected due to developmental and chemical concerns, the company addressed these issues and resubmitted the BLA, seeking accelerated approval in December 2019.
In April 2019, Immunomedics partnered with Everest Medicines for the development, registration, and commercialization of sacituzumab govitecan in Greater China, South Korea, and several Southeast Asian territories.
In April 2020, Trodelvy received fast-track designation from the FDA for the treatment of advanced or locally metastatic urothelial cancer. The same month, it gained accelerated approval for treating advanced triple-negative breast cancer (TNBC) in adult patients and was granted breakthrough therapy status.
In April 2021, the FDA approved Trodelvy as the first treatment for metastatic triple-negative breast cancer (TNBC). Later, in November 2021, Trodelvy gained approval in Europe for treating metastatic TNBC in patients who had undergone two or more prior systemic therapies.
In August 2022, Gilead Sciences secured the remaining global rights from Everest Medicines for the development and commercialization of Trodelvy in countries including China, South Korea, Singapore, Indonesia, the Philippines, Vietnam, Thailand, Malaysia, and Mongolia.
By February 2023, Trodelvy received FDA approval for pre-treated HR+/HER2- metastatic breast cancer in adult patients who had already received endocrine-based therapy and at least two additional systemic treatments. This approval was mirrored in Europe in July 2023.
Today, Trodelvy is approved in over 40 countries, solidifying its role in advanced cancer treatment worldwide.
How Trodelvy is Effective for Metastatic Triple-negative Breast Cancer (mTNBC):
Researchers evaluated Trodelvy in a phase-3 clinical trial of 529 adults comparing Trodelvy to traditional chemo in previously treated patients to find out if Trodelvy would:
- Slow the progression of metastatic triple-negative breast cancer (mTNBC)
Help individuals with mTNBC live longe - How many people were in the trial: A total of 529 adults were previously diagnosed with triple-negative breast cancer that had progressed to other parts of the body (metastatic) or could not be removed by surgery.
- 267 adults received Trodelvy
- 262 adults received single-agent, or traditional, chemo.
Their healthcare provider selected the type of chemotherapy they received. Types of chemotherapy included eribulin, gemcitabine, capecitabine, and vinorelbine. - Who were the Participants: All patients had been previously diagnosed with mTNBC and had received 2 or more therapies for breast cancer. At least 1 of these therapies was specifically for metastatic breast cancer.
Additional criteria included:
Patients with known Gilbert’s disease or bone-only disease were not included.
12% of the patients in the study had cancer that had spread to their brain, which was previously treated and stable.
All patients previously received taxane treatment.
- How was Trodelvy Given: It is an intravenous (IV) infusion. In the clinical trial, patients who were treated with Trodelvy received 10 mg/kg on days 1 and 8 of a 21-day treatment cycle. Researchers determined when to stop this treatment depending on how patients were responding to and tolerating treatment.
Trodelvy Helped Patients Live Longer than Traditional Chemotherapy:
Median Progression-Free Survival: Half of those who were treated with Trodelvy lived without cancer progression almost 3x longer. This result is known as median progression-free survival (PFS), which shows how long a treatment stops the progression or spread of mTNBC in half of the individuals who take it.
- Half of those who were treated with Trodelvy lived with no signs/symptoms of cancer progression for nearly 5 months.
- Half of those who were treated with traditional chemotherapy (eribulin, vinorelbine, gemcitabine, or capecitabine) lived with no signs/symptoms of cancer progression for around 2 months.
- Median overall survival (OS): Half of those who were treated with Trodelvy lived about 2x longer. This result is known as median overall survival (OS), which shows how long half of the individuals are alive after initiating treatment
- Half of those who were treated with Trodelvy lived nearly 12 months.
- Half of those who were treated with traditional chemotherapy lived for nearly 7 months.
How Trodelvy is Effective for Metastatic Bladder Cancer?
Researchers evaluated Trodelvy in a phase-2 clinical trial of 112 individuals with previously treated bladder cancer and cancers of the urinary tract that spread (metastatic) or could not be eliminated by surgery. They aimed to find out:
- How many individuals responded to treatment with Trodelvy?
- How long do the effects of Trodelvy last?
- Who were the participants: The trial included a total of 112 adults. Everyone enrolled in the trial had previously been diagnosed with bladder cancer and cancers of the urinary tract that spread or could not be removed by surgery. All participants had previously received platinum-containing chemotherapy and an immunotherapy medicine.
- How was Trodelvy Given: It is an intravenous (IV) infusion. In the clinical trial, individuals who were treated with Trodelvy received 10 mg/kg on days 1 and 8 of a 21-day treatment cycle. Researchers determined when to stop this treatment on behalf of how patients were responding to and tolerating treatment.
Trodelvy May Help Your Tumors Shrink or Disappear: A total of 27.7% of individuals (31 out of 112) responded to Trodelvy in the clinical trial.
22.3% (25 out of 112) saw their tumors reduce in size by at least 30%
5.4% (6 out of 112) saw their tumors disappear
The median† length of response to treatment with Trodelvy was 7.2 months (for 31 out of 112)
How Trodelvy is Effective for HR+/HER2- Metastatic Breast Cancer?
Researchers evaluated Trodelvy in a phase 3 clinical trial of a total of 543 adults with previously treated HR+/HER2- mBC comparing Trodelvy to traditional chemo to find out how long individuals:
- Lived without HR+/HER2- mBC progressing
- Lived with HR+/HER2- mBC overall
How many people were in the trial?
A total of 543 individuals with previously treated HR+/HER2- breast cancer that had progressed to other parts of the body (metastatic) or could not be removed by surgery.
- 272 individuals received Trodelvy
- 271 individuals received single-agent, or traditional, chemo
- Their researchers selected the type of chemotherapy they received. Types of chemotherapy included eribulin, gemcitabine, capecitabine, or vinorelbine.
Who were the participants: All patients had been previously diagnosed with HR+/HER2- mBC and had received:
- endocrine therapy
- CDK4/6 inhibitor (a
- type of targeted therapy)
- taxane (a type of chemotherapy) at least 2 and no more than 4 prior chemotherapies for
- metastatic breast cancer
One of the chemos could have been given as a neoadjuvant or adjuvant treatment if recurrence occurred within 12 months.
Note: Endocrine therapy, CDK4/6 inhibitor, and taxane treatments were used in a neoadjuvant, adjuvant, or metastatic setting.
How was Trodelvy Given?
- It is an intravenous (IV) infusion. In the clinical trial, those who were treated with Trodelvy received 10 mg/kg on days 1 and 8 of a 21-day treatment cycle. Researchers determined when to stop this treatment on behalf of how patients were responding to and tolerating treatment.
- It is an intravenous (IV) infusion. In the clinical trial, those who were treated with Trodelvy received 10 mg/kg on days 1 and 8 of a 21-day treatment cycle. Researchers determined when to stop this treatment on behalf of how patients were responding to and tolerating treatment.
- Trodelvy Helped Patients Live Longer than Traditional Chemotherapy:
Median Progression-Free Survival: Half of those treated with Trodelvy lived without cancer progression about 38% longer. This result is known as median progression-free survival (PFS), which indicates how long a therapy stops the progression or spread of HR+/HER2- mBC in half of the individuals who take it. - Half of those who were treated with Trodelvy lived with no sign of cancer progression for 5.5 months.
Half of those who were treated with traditional chemo (eribulin, vinorelbine, gemcitabine, or capecitabine) lived with no signs/symptoms of cancer progression for 4 months.
Median Overall Survival: Half of those who were treated with Trodelvy lived about 29% longer overall. This result is known as median overall survival (OS), which indicates how long half of the individuals are alive after initiating treatment. - Half of those who were treated with Trodelvy lived for over 1 year (14.4 months).
Half of those who were treated with traditional chemotherapy lived for 11.2 months.
FAQs for Trodelvy (sacituzumab govitecan-hziy)
How can patients avoid counterfeit medicines?
To avoid counterfeit medicines, patients should only purchase drugs from licensed pharmacies or trusted sources like the Indian Pharma Network (IPN), which ensures access to genuine medicines.
What is Trodelvy used for?
Trodelvy is an FDA-approved antibody-drug conjugate (ADC) used to treat advanced triple-negative breast cancer (TNBC) in adults who have undergone at least two prior treatments for metastatic disease. It is also approved for pre-treated HR+/HER2- metastatic breast cancer and locally advanced or metastatic urothelial cancer (mUC) in patients who have previously received platinum-based chemotherapy and a PD-1 or PD-L1 inhibitor.
How does Trodelvy work for metastatic triple-negative breast cancer (mTNBC)?
Trodelvy targets the Trop-2 protein present in cancer cells. It delivers a chemotherapy agent directly to these cells, reducing their growth and spread. In clinical trials, Trodelvy has been shown to slow the progression of mTNBC and extend overall survival compared to traditional chemotherapy.
What are the benefits of Trodelvy compared to traditional chemotherapy?
Trodelvy has demonstrated superior efficacy compared to traditional chemotherapy in treating metastatic TNBC. Patients receiving Trodelvy lived without cancer progression for nearly 5 months, compared to 2 months with traditional chemotherapy. Additionally, Trodelvy improved overall survival, with patients living about 12 months on average, compared to 7 months for those on traditional chemotherapy.
What are the common side effects of Trodelvy?
Common side effects of Trodelvy include nausea, diarrhea, fatigue, decreased appetite, and hair loss. As with any cancer treatment, individual experiences may vary, and it is important to discuss any side effects with your healthcare provider to manage them effectively.
How can I buy Trodelvy in India, and which cities it can be made available?
Patients, Clinicians, or Hospitals can buy Trodelvy online in India through authorized channels, such as the Indian Pharma Network (IPN), which facilitates its availability in the country. Trodelvy can be made available in major cities like Mumbai, Delhi, Bengaluru, Hyderabad, Chennai, Kolkata, Pune, Lucknow, Ahmedabad, Jaipur, Chandigarh, Bhopal, Patna, Surat, and Nagpur. For more details or to order, reach out to IPN via their TOLL-FREE helpline: 1800-889-1064 or Call/WhatsApp: +91 9310090915.