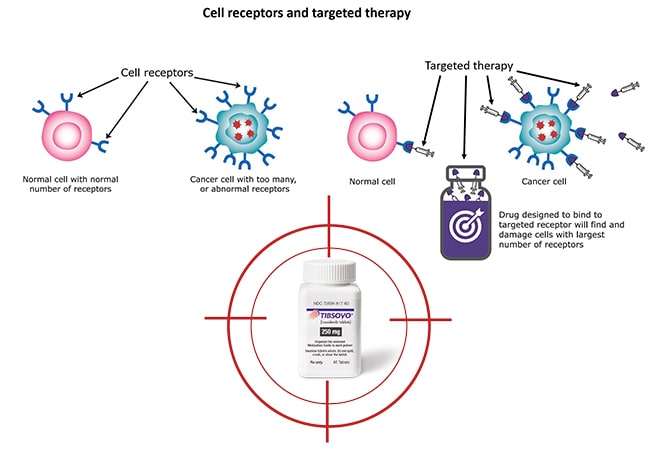
TIBSOVO (ivosidenib) is a groundbreaking prescription medication specifically designed for adults with an isocitrate dehydrogenase-1 (IDH1) mutation. This targeted therapy represents a significant advancement in the treatment of certain types of cancer, providing new hope for patients with limited options.
TIBSOVO has received approval for three key indications:
- Acute myeloid leukemia (AML)
- Myelodysplastic syndromes (MDS)
- Cholangiocarcinoma (bile duct cancer).
Acute Myeloid Leukemia (AML):
AML is a fast-progressing blood cancer characterized by the rapid growth of abnormal white blood cells, which accumulate in the bone marrow and interfere with normal blood cell production. AML is particularly aggressive and often difficult to treat, especially in older adults or those with underlying health conditions.
- TIBSOVO has shown promise in treating AML, particularly for patients with an IDH1 mutation.
- For newly diagnosed AML, TIBSOVO can be used in combination with azacitidine or as a monotherapy for individuals who are 75 years or older or who have comorbidities that preclude the use of intensive chemotherapy.
- This offers a less toxic alternative for vulnerable patients, enhancing their quality of life and potentially extending survival. Additionally, TIBSOVO is approved for AML that has relapsed or has not responded to prior treatments, providing a critical option for patients facing limited choices.
Myelodysplastic Syndromes (MDS):
MDS is a group of disorders caused by poorly formed or dysfunctional blood cells. These syndromes can progress to AML and are often challenging to manage. Treatment typically involves supportive care and medications to improve blood cell counts, but effective targeted therapies have been lacking.
- For patients with MDS who have an IDH1 mutation and whose disease has relapsed or has not improved after previous treatments, TIBSOVO offers a new therapeutic approach.
- By specifically targeting the mutated IDH1 enzyme, TIBSOVO helps to restore normal cell differentiation and reduce the number of immature blood cells. This can lead to improved blood counts, reduced transfusion needs, and potentially delay the progression to AML.
Cholangiocarcinoma (Bile Duct Cancer):
Cholangiocarcinoma is a rare and aggressive cancer that occurs in the bile ducts, which are part of the digestive system. The prognosis for advanced cholangiocarcinoma is generally poor, with limited treatment options available.
- TIBSOVO provides a new option for patients with advanced cholangiocarcinoma who have an IDH1 mutation and have previously received treatment.
- The approval of TIBSOVO for this indication is particularly significant because it offers a targeted therapy option where few effective treatments existed.
- By inhibiting the mutated IDH1 enzyme, TIBSOVO can help to slow the progression of the cancer, providing patients with more time and potentially improving their quality of life.
The Importance of IDH1 Testing:
Before starting TIBSOVO, it is essential for patients to undergo genetic testing to confirm the presence of an IDH1 mutation. This ensures that the treatment is appropriate and likely to be effective. The role of precision medicine in cancer treatment is increasingly important, as it allows for therapies to be tailored to the unique genetic makeup of an individual’s cancer.
Safety and Effectiveness:
- While TIBSOVO represents a significant advancement in cancer treatment, its safety and effectiveness in children have not been established.
- As with any medication, patients taking TIBSOVO should be monitored for potential side effects, which can include fatigue, nausea, diarrhea, and changes in blood cell counts. Regular follow-ups with healthcare providers are crucial to managing these effects and adjusting treatment as needed.
TIBSOVO marks a significant milestone in the field of oncology, offering new hope for patients with AML, MDS, and cholangiocarcinoma. Its targeted approach, focusing on the IDH1 mutation, exemplifies the potential of precision medicine to transform cancer treatment. As research continues and more data become available, TIBSOVO may pave the way for even more innovative therapies, ultimately improving outcomes for patients with these challenging cancers.
What is Tibsovo, and how does it work?
Tibsovo (ivosidenib) is a targeted therapy designed to inhibit the mutated IDH1 enzyme found in certain cancers. By blocking this enzyme, this therapeutic drug helps restore normal cell differentiation and reduce the proliferation of cancerous cells.
Which cancers are treated with Tibsovo?
Tibsovo is approved for the treatment of acute myeloid leukemia (AML) with an IDH1 mutation, myelodysplastic syndromes (MDS) with an IDH1 mutation, and advanced cholangiocarcinoma (bile duct cancer) with an IDH1 mutation.
How is Tibsovo administered?
Tibsovo is administered orally in tablet form. The dosage and treatment regimen depend on the specific condition being treated and the patient’s overall health status.
What are the common side effects of Tibsovo?
If you are looking to buy Nelarabine injections for your healthcare facility or patients, we offer a reliable solution. We ensure that no region is left underserved, meeting global demands for this critical medication. With IPN, you can expect timely deliveries, professional services, and adherence to the highest quality standards.
Is Tibsovo available in India, and how can patients access it?
As of now, Tibsovo is not registered in India. However, patients can access it through legal channels such as the Named Patient Program, which allows the import of unregistered medications for personal use under specific conditions. Patients should consult with their healthcare provider and authorized service providers to facilitate this process.
How can I buy Tibsovo in major cities across India and internationally?
Tibsovo can be procured in major Indian cities. by Indian Pharma Network (IPN). Send your inquiry via Call/WhatsApp: +91 9310090915 to buy Tibsovo in New Delhi, Gurgaon, Noida, Lucknow, Mumbai, Chennai, Bengaluru, Hyderabad, Kolkata, Pune, Ahmedabad, and Jaipur through authorized suppliers specializing in importing unregistered medicines. Additionally, international patients from Canada, Australia, UAE, Saudi Arabia, Singapore, Malaysia, South Africa, Brazil, France, Germany, Italy, Spain, and Japan can access Tibsovo through legal supply channels. Consult with healthcare providers and trusted networks like IPN to facilitate the process.
Is Tibsovo available in SAARC countries, and how can patients access it?
Yes, Tibsovo can be accessed in SAARC countries, including India, Afghanistan, Bangladesh, Bhutan, Maldives, Nepal, Pakistan, and Sri Lanka, through authorized suppliers or the Named Patient Program. Patients in these countries should consult with their healthcare providers and trusted services like IPN to ensure proper documentation and legal importation.