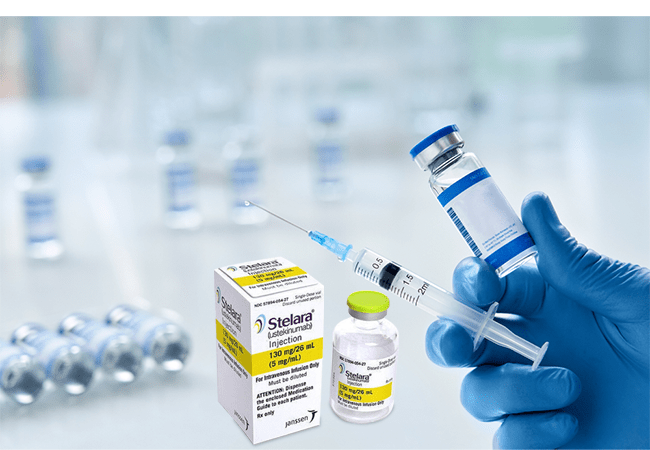
About Ustekinumab: The Ustekinumab comes under the brand name Stelara. It is mainly a monoclonal antibody. Monoclonal antibodies are specific proteins that identifies and bind to certain proteins in the body. Ustekinumab belongs to a group of medications known qs ‘immunosuppressants’. These medications act in order to weaken the part of the immune system.
- Indication: The ustekinumab injection is aimed to treat the certain following inflammatory diseases:
- Plaque Psoriasis: in adults as well as children aged 6 years and older.
- Psoriatic Arthritis: in adult patients.
- Moderate-Severe Crohn’s disease: in adult patients.
- Moderate-Severe UC (Ulcerative Colitis): in adults patients.
Dosage: The ustekinumab 45 mg injection is intended for use under the close guidance and supervision of a specialist experienced in order to treat conditions for which ustekinumab is intended.
Adults aged 18 years or older with Psoriasis or Psoriatic Arthritis: The standard starting ustekinumab dose is 45 mg. Patients who weigh more than 100kg can start on a dose of 90mg instead of 45mg. Following the starting dose, patient will have the next dose 4 weeks later, and then every 12 weeks.
Adults aged 18 years or older with Crohn’s disease or Ulcerative Colitis: During therapy, the first stelara dose of approximately 6 mg/kg will be administered by a healthcare provider through intravenous infusion. Following the starting dose, patient will have the next dose of 90 mg after 8 weeks, then every 12 weeks thereafter subcutaneously. In some patients, following the first injection under the skin, 90 mg can be administered every 8 weeks.
Children/Adolescents aged six years or older with Psoriasis: Healthcare profesaional will work out the appropriate dose, including the volume of stelara injection to be injected to give the apt dose. The right dose will depend on patient’s body weight at the time each dose is administered. A 45 mg vial is available for children who need to have less than the full 45mg dose. If patients weigh less than 60kg, the standard dose is 0.75 mg per kg body weight. If patients weigh 60-100 kg, the standard dose is 45 mg. If patients weigh more than 100 kg, the standard dose is 90 mg. After the starting dose, patients will have the next dose 4 weeks later, and then every 12 weeks.
Administration: Ustekinumab should always be used exactly as per the words told by a healthcare professional. Discuss with doctor about when you will receive your injections and follow-up appointments. The stelara 45 mg is administered as an injection under the skin (‘subcutaneously’). At the beginning of therapy, anyone from healthcare team may inject this medication. Although, you and your specialist may decide that you may inject this medicine yourself. In this case you will get the training on how to inject it yourself.
Possible Side Effects: Ustekinumab may cause serious side effects, including:
Serious allergic reactions: Serious allergic reactions may occur with Ustekinumab. Stop using this drug and get immediate medical assistance in case you have the symptoms of serious allergic reactions including feeling faint, chest tightness, skin rash and swelling of face, tongue, eyelids and throat.
Lung inflammation: In some cases, lung inflammation may happen in recipients who receive Ustekinumab, and this may be serious. These lung complications may require to be treated. Report your healthcare professional promptly in case you have shortness of breath or a cough that is persistent during treatment with Ustekinumab.
Common ustekinumab side effects include:
- Nasal congestion, sore throat, and runny nose
- Redness at the injection site
- Upper respiratory infections
- Vaginal yeast infections
- Fever
- Urinary tract infections
- Headache
- Sinus infection
- Tiredness
- Bronchitis
- Itching
- Diarrhea
- Nausea and vomiting
- Stomach pain
- The above listed are not the all possible side effects of Ustekinumab. Contact your doctor or healthcare provider for medical instructions about side effects.
Pregnancy and breast-feeding: It is preferable to avoid the ustekinumab uses in pregnancy. The effects of this medication in pregnant women are unknown. In case you’re a woman of childbearing age, you are advised to avoid
becoming pregnant and must consider the apt contraception while using ustekinumab and for minimum 15 weeks following the last dose of treatment. Interact with your doctor if you are pregnant, think you could be pregnant or are planning to conceive a baby. Discuss with your healthcare team if you’re breast-feeding or are planning to breast-feed. You along with your specialist should decide in case you need to breast-feed or use ustekinumab -do not do both.
Driving and using machines: Ustekinumab has no or negligible influence on the capability to drive and use machines.
Overdose: In case you have used or have been administered too much Ustekinumab, talk to a specialist straight away. Always have the outer carton of the medication with you, even if it is empty.
Missed Dose: If you forget ustekinumab 45 mg injection dose, contact your healthcare team. It is not recommended to take a double dose for making up a forgotten dose.
Storage: Store Ustekinumab vials and prefilled syringes in a refrigerator between 36°F to 46°F (2°C to 8°C). Vials should be stored standing up straight. Store this medication in the original carton to protect it from light until time to use it. Neither freeze nor shake Ustekinumab.
Price: The stelara injection cost in India can vary. To know the exact stelara price as well as procurement details, kindly get in touch with us today at our TOLL-FREE No: 1800-889-1064.
Note: The information provided in the article named “Ustekinumab: Medication Guide”, is just for informational purposes and it is not responsible for serving as a substitute for the medical treatment, consultation, diagnosis, of an experienced healthcare professional.
Where can I buy Ustekinumab online in India?
You can legally buy Ustekinumab online in India through the Indian Pharma Network (IPN). We ensure the legal supply of genuine imported medicines, including Stelara, to patients across India and other countries. Contact us at +91 9310090915 to know the procedure to procure this therapeutic drug.
Which countries offer access to Ustekinumab?
Ustekinumab (Stelara) can be made available in several countries, including UAE, Sri Lanka, Bangladesh, Japan, South Korea, Pakistan, Afghanistan, Singapore, and Qatar. Indian Pharma Network facilitates the legal supply of Ustekinumab to these and other countries.
How can I access Ustekinumab in India?
Ustekinumab (Stelara) is an imported medicine available in India for treating psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. IPN specializes in sourcing and delivering Ustekinumab across India. Call +91 9310090915 to learn more.
Why is Ustekinumab considered an imported medicine?
Ustekinumab is classified as an imported medicine in India because it is manufactured and approved in countries like the United States and Europe before being brought to India. Its availability depends on global sourcing, and IPN ensures a seamless supply chain.
What makes Indian Pharma Network a trusted partner for Ustekinumab in India?
Indian Pharma Network bridges the gap for patients in need of imported medicines like Ustekinumab. By working with licensed distributors, we ensure timely delivery, genuine products, and compliance with all regulations.