The U.S. FDA has approved sacituzumab govitecan, marketed as Trodelvy, for treating advanced hormone receptor (HR)-positive, HER2-negative breast cancer. This approval is monumental for Indian patients and healthcare providers (HCPs), as it expands the drug’s application beyond its initial use for treating triple-negative breast cancer. Trodelvy offers renewed hope, especially for those with few remaining treatment options.
Understanding HR-Positive/HER2-Negative Breast Cancer:
HR-positive/HER2-negative breast cancer is the most common subtype of breast cancer. Early detection and localized tumors usually lead to high survival rates, with nearly all patients living at least five years post-diagnosis. In India, where breast cancer cases are rising, this subtype accounts for a significant portion of diagnoses. For tumors that have spread locally, the five-year survival rate is over 90%. However, survival drops to about 32% for patients whose cancer has metastasized to distant organs, emphasizing the need for advanced treatments like Trodelvy.
How Trodelvy Works?
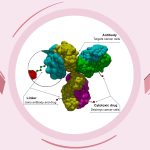
Trodelvy, developed by Gilead Sciences, belongs to a class of drugs known as antibody-drug conjugates (ADCs). These drugs combine a chemotherapy agent with an antibody that targets specific cancer antigens, allowing for precise and effective treatment. For Indian patients with advanced HR-positive/HER2-negative breast cancer that hasn’t responded to prior chemotherapy or endocrine treatments, Trodelvy offers a new option. By targeting cancer cells more effectively, it helps slow tumor growth, improve symptoms, and potentially extend survival.
Results from the TROPiCS-02 Trial:
The TROPiCS-02 trial, which included 543 patients with advanced HR-positive/HER2-negative breast cancer, compared Trodelvy with standard chemotherapy. The findings were promising:
Progression-Free Survival (PFS): Patients treated with Trodelvy experienced a median progression-free survival of 5.5 months, compared to 4 months for those on chemotherapy.
Overall Survival (OS): Median overall survival for Trodelvy patients was 14.4 months, versus 11.2 months for chemotherapy patients.
While both groups experienced side effects, neutropenia, and diarrhea were more common among Trodelvy users. However, experts believe the benefits outweigh the risks, as Trodelvy offers a new line of defense against advanced cancer.
The Impact of Trodelvy in India:
For patients in India, where access to advanced cancer treatments has been a challenge, Trodelvy’s approval is a critical development. With breast cancer cases expected to rise, effective therapies like Trodelvy can make a significant difference, especially for patients who have exhausted other options. This drug has the potential to extend survival and enhance the quality of life for patients, offering a hopeful option for advanced HR-positive/HER2-negative breast cancer.
Expert Opinions on Trodelvy’s Potential:
Dr. Kiven Erique Lukong, a breast cancer researcher, calls Trodelvy a “game-changer” for patients who have tried every other form of treatment. He notes that the drug can help some patients live longer, reduce symptoms, and slow tumor growth. Professor Hope Rugo, a renowned breast oncologist, adds that Trodelvy gives patients a new option when other therapies have been exhausted, allowing them to continue their treatment journey with improved well-being.
A Promising Future with Trodelvy:
Trodelvy has emerged as a hopeful option for Indian breast cancer patients facing advanced HR+/HER2- breast cancer. As a unique antibody-drug conjugate, it combines the precision of targeted therapy with the power of chemotherapy, helping patients achieve better outcomes. For those struggling with limited treatment choices, Trodelvy’s approval offers a new lease on life and a chance to live with improved quality.
For more information on how to buy Trodelvy online in India, contact Indian Pharma Network (IPN) via Call/WhatsApp: +91 9310090915 or TOLL-FREE: 1800-889-1064.
Frequently Asked Questions (FAQs):
What makes Trodelvy different from other breast cancer treatments?
Trodelvy is an antibody-drug conjugate that combines targeted therapy with chemotherapy, specifically attacking cancer cells. This targeted approach reduces damage to healthy cells, making it more effective for advanced cases.
Who can benefit most from Trodelvy?
Trodelvy is designed for patients with advanced HR-positive/HER2-negative breast cancer who have not responded to previous treatments like chemotherapy or hormonal therapy. It offers a new option for patients in need of effective, advanced treatment.
How does Trodelvy compare with chemotherapy in terms of survival rates?
In clinical trials, Trodelvy extended progression-free survival to 5.5 months, compared to 4 months with chemotherapy. The overall survival was also higher with Trodelvy, with a median of 14.4 months versus 11.2 months for chemotherapy.
Are there side effects associated with Trodelvy?
Yes, like many cancer treatments, Trodelvy has side effects, including neutropenia (low white blood cell count) and diarrhea. However, for many patients, the potential benefits of extended survival and symptom relief outweigh these risks.
How can Indian patients access Trodelvy?
Trodelvy can be accessed in India through trusted suppliers. For assistance on how to buy Trodelvy in India and other countries, patients can contact the Indian Pharma Network (IPN) for guidance on accessing the drug through legal channels.