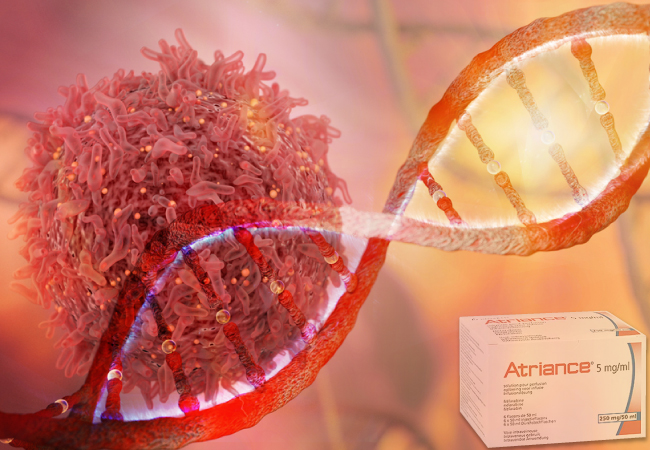
Nelarabine Injection, a ground-breaking oncology medicine intended to treat T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma in adult and pediatric patients aged one year and above, has made its momentous commercial debut, as announced by Shorla Oncology and EVERSANA. This advancement is especially noteworthy because T-cell leukemia, an aggressive blood and bone marrow malignancy primarily affecting adolescents, previously lacked access to effective treatments.
The FDA approval of Atriance Injection in March 2023 paved the way for its availability through major wholesalers and speciality distribution partners. This medical advancement provides a crucial alternative to existing therapies and gives patients hope if their diseases have not improved or have worsened after receiving at least two chemotherapy treatments. Shorla Oncology and EVERSANA hope to significantly enhance patient outcomes and lessen the strain on clinicians who struggle to find efficient therapies for their patients by offering a new therapy option.
As the first product authorized for the American market, the commercial launch represents a significant accomplishment for Shorla Oncology. Shorla’s pipeline shows promise for addressing unmet patient needs as a pharmaceutical business specializing in creating novel oncology treatments focusing on orphan and pediatric cancers. The partnership with EVERSANA guarantees thorough launch support, including support for the 3PL channel, trade relations, medical information, pharmacovigilance, quality services, revenue management, and sales and training solutions.
Shorla Oncology’s CEO and co-founder, Sharon Cunningham, emphasized her company’s delight in providing Nelarabine Injections to doctors nationwide. Medication availability is anticipated to benefit patients, particularly children, fighting leukemia. One of the critical strategies for improving this novel therapy’s uptake and patient outcomes is educating the clinical and patient communities about it.
Nelarabine Injection’s release heralds a potential step in oncology study and development. The FDA’s approval of the drug creates a precedent for future advancements in treating T-cell leukemia. It gives patients and their families who are facing restricted treatment options hope. Shorla Oncology and EVERSANA’s joint initiatives are prime examples of how industry alliances can hasten the accessibility of life-saving therapies and meet critical medical requirements. While the oncology community celebrates this achievement, attention now turns to ensuring the safe and effective use of nelarabine injection, with its crucial safety information that must be appropriately conveyed to patients and healthcare providers. This commercial launch is a tribute to the effectiveness of creativity, commitment, and teamwork in developing cancer treatment and enhancing patient outcomes.
Reference–
https://www.eversana.com/2023/05/16/shorla-oncology-eversana-announce-commercial-launch-of-recent-fda-approved-nelarabine-injection-for-the-treatment-of-t-cell-leukemia-across-the-united-states/
https://www.prnewswire.com/news-releases/shorla-oncology–eversana-announce-commercial-launch-of-recent-fda-approved-nelarabine-injection-for-the-treatment-of-t-cell-leukemia-across-the-united-states-301825409.html