According to the India Brand Equity Foundation, The Indian pharma sector has a long history of developing and delivering top-class products at reasonable costs across the globe. India is known as the ‘pharmacy of the world’ and ranked third-largest in pharma production by volume and 14th-largest by value. India has the most United States Food and Drug Administration-approved units (741 as of August 2021).
Instead of being one of the largest pharmacies in the World, India needs to import numerous medicines from other countries under the Named Patient Import Program. Name Patient Program is focused on delivering or dispensing medications under Orphan drugs, pre-approved drugs, Unlicensed Drugs, or Special drugs to patients and doctors to assist rare disease treatment or treatment under exceptional conditions.
The Named Patient Program (NPP) has accessibility to all well-established pharma- markets and sponsors for sourcing the necessary medications. Such programs ensure patients and doctors safe dispensing of the drug for appropriate use. The Named Patient Program (NPP) brings the global market under one umbrella for physicians, patients as well as sponsors. Name Patient Program maintains all the regulatory and logistics requirements to provide hassle-free service to all consumers.
The patient from any country recognized as ‘the Named Patient’ and suffering from a chronic, serious, or life-threatening disease has the right to access, procure, and import medicines that have life-saving potentiality or improve the quality of life of the patient.
But rules and regulations for importing innovative medications are different in every country. Every country has to follow its regulation to import such medications. Different programs already existed, such as Named Patient Import, Early Access Program, and Compassionate Use Program, specifically available to import such medications. However, every country follows its program.
In India import, manufacturing, sale, and distribution of drugs are regulated under the Drugs and Cosmetics Act 1940 and Drugs and Cosmetic Rules 1945, and New Drugs and Clinical Trials Rules 2019.
Indian Pharma Network (IPN) is one of the top certified pharmaceutical organizations in India which facilitates Named Patient Import (NPI) with a mission to be a portal for every single individual out there, no matter who they are or where they are based, has identical, fast and fair access to the advanced and best healthcare. We work on individual patients, on a Named Patient import basis, per their treating healthcare professional. There are several medicines, such as Tuksya, Trodelvy, Braftovi, Retevmo, Kineret , Zelboraf, Blincyto, Zavesca etc., which IPN serves under NPI.
We (IPN) favor them in accessing life-saving medicines when particular needs are fulfilled:
- The life-saving medication has market approval in another nation and is unapproved or unavailable in their own nation.
- There is no alternative on the market.
- The medication is for personal use.
- The respective patient has a valid prescription letter from their doctor in their home country.
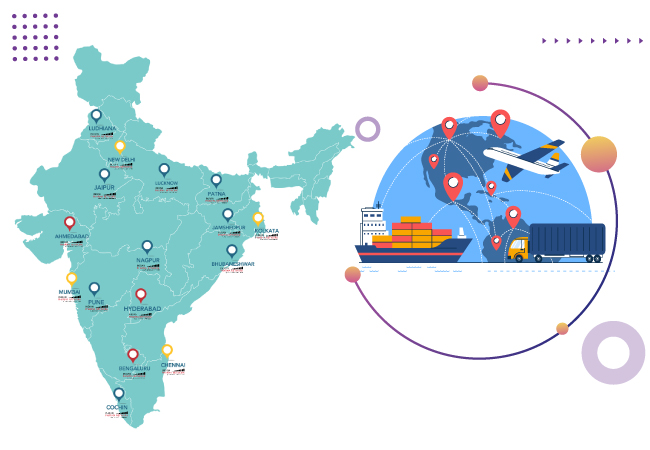
IPN serves every corner of India, such as Tier 1 cities, namely: Delhi, Mumbai, Bengaluru, Ahmedabad, Chennai, Kolkata, etc., and in Tier 2 cities, namely: Visakhapatnam, Guwahati, Patna, Chandigarh, Raipur, Ahmedabad, Surat, Gurugram, Faridabad, Shimla, Mysore, Kochi, Bhopal, Gwalior, Ujjain, Bhubaneswar, Ludhiana, Jaipur, Coimbatore, Vellore, Agra, Lucknow, Noida, Dehradun, Siliguri, etc.
In the Named Patient Service we provide, we aim to encourage the patient to consult their healthcare professional to establish their best course of treatment. Once we get a valid prescription letter for that treatment, we confirm the validity of the information given, then manage all the following steps; from sourcing to shipping to customs and delivery, as well as following up to make sure that everything was received in an appropriate order.