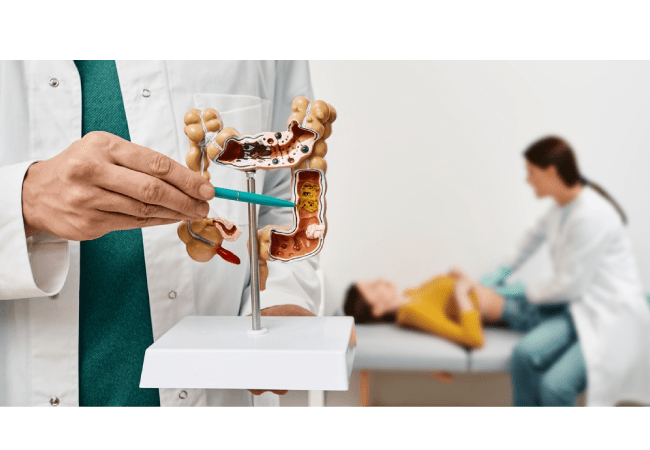
The FDA has approved a new treatment for some patients with advanced colorectal cancer. This treatment works for those with a specific marker called HER2-positive, found in a small percentage of colorectal cancer cases. It’s a combination therapy, meaning it uses two drugs together. This approval means patients meeting certain criteria can now access this treatment, offering hope for those with this type of cancer. HER2-positive was first identified in breast cancer but is also found in a small number (about 3% to 5%) of colorectal cancer cases.
Medical Information:
The FDA approved a new combination of two drugs, Tukysa and Herceptin, to treat a serious condition called HER2-positive cancer. Tukysa comes in pill form and targets HER2, while Herceptin is given through an IV and has been used for breast and stomach cancers. The advantage of this combo is that neither drug is chemotherapy; they’re targeted therapies. Targeted therapies deliver toxins to kill cancer cells without hurting healthy ones, offering a more precise treatment option. This approval allows patients to access these drugs sooner, addressing an urgent medical need.
Who is the Treatment for?
This new treatment combination is only for patients who have all of these:
- RAS Wild-type
- HER2-positive metastatic colorectal cancer
- Have advanced colorectal cancer that can’t be removed with surgery or has spread to other parts of your body.
- Have had chemotherapy with drugs like 5-FU, capecitabine, irinotecan, and oxaliplatin before, you might be eligible for this treatment.
Tukysa and Herceptin together are now approved by the FDA to treat advanced colon cancer with the HER2 gene mutation.
Is the Treatment Safe and Promising?
The MOUNTAINEER study, conducted under rigorous clinical trial protocols, demonstrated the safety and effectiveness of this treatment combination. Leading scientists provided compelling evidence from phase 2 of the trial to the FDA, resulting in expedited approval. The research targeted individuals with HER2-positive metastatic colorectal cancer who had prior chemotherapy. This promising study offers hope to eligible patients.
Findings from the MOUNTAINEER study revealed that:
- On average, patients experienced improvement in their condition for approximately 12.4 months, ranging from 8.5 to 20.5 months.
- On average, the period without disease progression lasted around 8.2 months, ranging from 4.2 months to 10.3 months.
- On average, patients survived for approximately 24.1 months, with individual survival times ranging from 20.3 months to 36.7 months.
Upon entering the study, approximately 64.3% of patients had liver metastases, and around 70.2% had lung metastases. Before the study, these patients had undergone an average of three treatment cycles, ranging from one to six cycles.
Seagan offered patient support for both insured and uninsured individuals participating in the clinical trial. Results revealed a notable decrease in tumor size and prolonged treatment effectiveness compared to standard care.
Reference:
https://www.cancer.gov/news-events/cancer-currents-blog/2023/fda-tucatinib-her2-colorectal-cancer