Important Facts About Trodelvy:
Drug Name: Trodelvy (Sacituzumab govitecan-hziy)
FDA Approval: Yes (First approved April 22, 2020)
Indication: Trodelvy is an FDA-approved medication for treating metastatic triple-negative breast cancer (mTNBC), HR-positive/HER2-negative breast cancer, and urothelial cancers, including bladder cancer that has spread or cannot be surgically removed. In India, this therapy is being used as an advanced treatment option for these aggressive cancers.
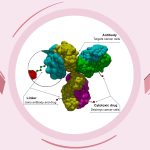
Mechanism of Action: Trodelvy is an antibody-drug conjugate (ADC) targeting Trop-2, a protein highly expressed in many cancer types, including breast and bladder cancers. Trodelvy links an antibody to SN-38, a chemotherapy agent. This combination delivers the chemotherapy directly to the cancer cells, reducing damage to healthy cells and improving overall efficacy.
Efficacy: Recent clinical trials have confirmed Trodelvy’s effectiveness, especially for patients with metastatic triple-negative breast cancer, showing improvements in both progression-free survival (PFS) and overall survival. A study of mTNBC patients in India demonstrated significant life extension and symptom relief. The HR-positive/HER2-negative breast cancer patients and those with advanced urothelial cancers have also experienced improved outcomes with Trodelvy.
Administration: Trodelvy is administered intravenously (IV) in cycles as prescribed by your healthcare provider. Each dosage and frequency is carefully determined based on individual health conditions.
Side Effects: Common side effects include fatigue, nausea, vomiting, diarrhea, hair loss, decreased appetite, and anemia. Some serious side effects may include neutropenia (low white blood cell count), febrile neutropenia (neutropenia with fever), and severe diarrhea. It’s crucial to inform your healthcare provider about any side effects you experience.
Monitoring: Regular monitoring of blood counts and liver function tests is important during Trodelvy treatment. In India, healthcare providers are ensuring close follow-up with patients to track side effects and adjust treatments as needed.
Cost: Trodelvy can be expensive. The cost may vary based on various factors like treatment cycles and dosage. Indian Pharma Network (IPN) offers pricing details and patient assistance programs to make medicine accessible in India. Contact IPN for the latest pricing and guidance on how to obtain Trodelvy.
Important Safety Information of Trodelvy:
Infusion Reactions: Trodelvy may cause infusion reactions such as chills, fever, shortness of breath, and wheezing. In India, clinicians advise patients to report these symptoms immediately to ensure timely intervention. Most reactions occur during or shortly after the infusion.
Severe Neutropenia: Trodelvy may significantly lower the white blood cell count, making patients more vulnerable to infections. Regular blood count monitoring is necessary throughout treatment to catch potential complications early. Indian healthcare providers often prescribe supportive therapies to prevent infections.
Diarrhea Management: Diarrhea is a frequent and serious side effect. Patients are advised to follow their doctor’s recommendations, including using anti-diarrheal medications and staying hydrated to manage this condition.
Interstitial Lung Disease (ILD): Although rare, ILD can occur, and it has been reported in patients receiving Trodelvy. Any new or worsening cough, shortness of breath, or fever should be reported to the doctor immediately.
Hematologic Toxicity: Patients undergoing treatment may face blood disorders such as thrombocytopenia (low platelet count) or severe anemia. Regular blood tests are conducted in India to catch and manage these risks early.
Embryo-Fetal Toxicity: Trodelvy can harm unborn babies, and it’s advised that women of childbearing age use effective contraception during treatment and for some time afterward to avoid pregnancy complications.
Frequently Asked Questions (FAQs):
What is Trodelvy, and how does it work?
Trodelvy (sacituzumab govitecan-hziy) is an advanced treatment for certain types of breast cancer and bladder cancer. It works by delivering a chemotherapy drug directly to the cancer cells through an antibody-drug conjugate (ADC) that targets the Trop-2 protein found in cancer cells.
What types of cancer is Trodelvy used to treat?
Trodelvy is approved to treat metastatic triple-negative breast cancer (mTNBC), HR-positive/HER2-negative breast cancer, and urothelial cancers, including bladder cancer, that have spread or cannot be surgically removed.
What are the common side effects of Trodelvy?
The common side effects include fatigue, nausea, vomiting, hair loss, diarrhea, decreased appetite, and low white blood cell counts. Serious side effects may include severe diarrhea and neutropenia.
How is Trodelvy administered?
Trodelvy is administered via intravenous (IV) infusion in cycles determined by your doctor based on your health condition. You may need multiple cycles depending on how you respond to the treatment.
How much does Trodelvy cost in India?
The cost of Trodelvy can be high, depending on the dosage and number of cycles required. To find the most up-to-date Trodelvy price in India and inquire about availability, contact the Indian Pharma Network (IPN) via Call/WhatsApp: +91 9310090915, which can help facilitate access to this medicine.