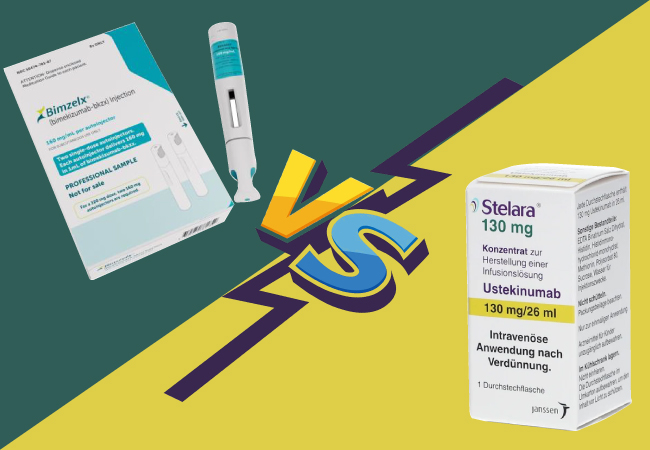
Bimzelx (bimekizumab-bkzx) is a newer biologic therapeutic drug that selectively blocks interleukin-17A and interleukin-17F, which are key drivers in inflammatory processes, and is approved for moderate to severe plaque psoriasis (Ps). Stelara (ustekinumab), on the other hand, is a biological medicinal product that targets interleukin-12 and interleukin-23, cytokines involved in inflammatory and immune responses, and is approved for moderate to severe plaque psoriasis (Ps), psoriatic arthritis (PsA), Crohn’s disease (CD), and ulcerative colitis (UC).
When making a decision between the two medicines, it’s crucial to take into account their specific uses, how they work, possible side effects, and guidance from a healthcare professional. Individual reactions and medical backgrounds play a significant role in determining the most suitable treatment.
Difference Between Bimzelx and Stelara:
Bimzelx (bimekizumab-bkzx):
- Generic Name: Bimekizumab-bkzx
- Indications: Plaque psoriasis
- Mechanism of Action: Interleukin-17A and interleukin-17F inhibitor
- Administrative Route: Subcutaneous
- Side Effects: Upper respiratory infections, headache, oral candidiasis, fatigue, injection site reactions
- Contraindications: Active tuberculosis, severe infections, hypersensitivity to bimekizumab-bkzx
- Drug Class: Monoclonal antibody
- Manufacturer: UCB
- Regulatory Agency Approvals: Food and Drug Administration (FDA), European Medical Agency (EMA)
Stelara (ustekinumab):
- Generic Name: Ustekinumab
- Indications: Plaque psoriasis, psoriatic arthritis, Crohn’s disease, ulcerative colitis
- Mechanism of Action: Interleukin-12 and interleukin-23 inhibitor
- Administrative Route: Subcutaneous, intravenous
- Side Effects: Upper respiratory infections, injection site reactions, headache, fatigue
- Contraindications: Active tuberculosis, severe infections, hypersensitivity to ustekinumab
Drug Class: Monoclonal antibody - Manufacturer: Janssen Biotech
- Regulatory Agency Approvals: Food and Drug Administration (FDA), European Medical Agency (EMA)
Efficacy of Bimzelx and Stelara:
Bimzelx (Bimekizumab-bkzx) for Psoriasis: Bimzelx, a newer biologic therapy, is now approved to treat moderate to severe plaque psoriasis in adults who need systemic therapy or phototherapy. It targets interleukin-17A (IL-17A) and interleukin-17F (IL-17F), which play crucial roles in causing psoriasis. Studies have shown that Bimzelx can significantly improve psoriasis symptoms. In Phase 3 trials called BE VIVID and BE READY, many patients saw impressive results within 16 weeks of starting treatment. They experienced at least 90% or 100% improvement in their PASI scores, which measure the severity of psoriasis. These improvements were maintained with continued treatment for up to 52 weeks.
Stelara (Ustekinumab) for Psoriasis: Stelara (ustekinumab) is a medication used to treat moderate to severe plaque psoriasis in adults who may benefit from phototherapy or other systemic treatments. By targeting interleukin-12 (IL-12) and interleukin-23 (IL-23), which are proteins involved in psoriasis-related inflammation, Stelara helps to improve skin symptoms. Clinical studies, including Phase 3 trials known as PHOENIX 1 and PHOENIX 2, have shown that Stelara can lead to significant skin improvement, with more than two-thirds of patients experiencing a 75% reduction in their Psoriasis Area and Severity Index (PASI 75) score after 12 weeks of treatment. Stelara’s effectiveness has also been demonstrated to persist over time with continued use.
Comparative Efficacy in Psoriasis Treatment: Although both Bimzelx and Stelara have proven highly effective in treating psoriasis, they work in different ways by targeting different cytokines involved in the disease’s development. As of now, there haven’t been direct head-to-head studies comparing the two medications, so it’s difficult to determine which one is superior. The decision between Bimzelx and Stelara may vary depending on individual factors such as the severity of the disease, how well previous treatments have worked, and any other health conditions the patient may have. Safety considerations and potential side effects should also be taken into account when making a treatment choice.
Conclusion:
Both Bimzelx and Stelara offer effective relief for moderate to severe plaque psoriasis, backed by clinical trials demonstrating significant improvements in skin clearance. Bimzelx, targeting IL-17A and IL-17F, is a newer class of biologic with promising results from studies. Meanwhile, Stelara, focusing on IL-12 and IL-23, boasts a longer history of use. Deciding between the two should involve consultation with a healthcare provider, considering the patient’s unique needs and medical background. Ongoing research and monitoring further enhance our understanding of how these treatments can effectively and safely manage psoriasis.
Access Bimzelx or Stelara Through Legal Channels:
If Bimzelx or Stelara are not approved or available in your home country, you can readily access these therapeutic drugs via Indian Pharma Network (IPN).
References:
https://www.stelarainfo.com/
https://pubmed.ncbi.nlm.nih.gov/33549193/
https://www.ajmc.com/view/bimekizumab-bkzx-the-newest-psoriasis-treatment-is-now-available