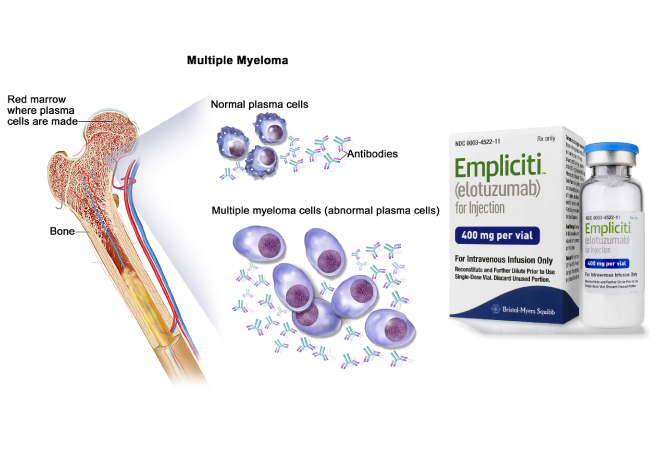
The US Food and Drug Administration (FDA) approved Empliciti (elotuzumab) for use in combination with medicines lenalidomide and dexamethasone for patients with multiple myeloma who have received 1 to 3 previous therapies.
Empliciti (elotuzumab) is the first monoclonal antibody designed to target the signaling lymphocytic activation molecule family (SLAMF) 7 protein, and the second monoclonal antibody authorized for those with relapsed multiple myeloma (MM). This approval was based on a 2-year analysis of the ELOQUENT-2 study, a randomized phase 3 clinical trial that showed a 30% improvement in progression-free survival (PFS) when the medicine elotuzumab was added to lenalidomide and dexamethasone compared with lenalidomide plus dexamethasone alone.
Mechanism of Action:
Empliciti (elotuzumab) is a humanized immunoglobulin (Ig) G1 monoclonal antibody that targets the SLAMF7 protein. SLAMF7 is a receptor that exists on immune cells, including natural killer cells, plasma cells, and specific immune-cell subsets of differentiated cells within the hematopoietic lineage.
The medicine is also expressed on multiple myeloma (MM) cells regardless of cytogenetic abnormalities. It directly activates natural killer cells with the help of the SLAMF7 pathway and Fc receptors on immune cells. This therapeutic drug targets SLAMF7 on multiple myeloma (MM) cells and facilitates interactions with natural killer cells to mediate the destruction of tumor cells through antibody-dependent cellular cytotoxicity.
Dosing and Administration:
The proposed dose of elotuzumab, when used along with lenalidomide and low-dose dexamethasone, is 10 mg/kg administered intravenous infusion weekly for the first 2 cycles and every 2 weeks thereafter until disease progression or until unacceptable toxicity. Therapy with the elotuzumab-based regimen needs to be continued until disease progression or until unacceptable toxicity.
Premedicate patients with 4 agents, including dexamethasone; an H1 blocker, such as diphenhydramine; an H2 blocker, such as ranitidine; and acetaminophen given 4-90 minutes before the infusion of elotuzumab.
Elotuzumab is supplied in 300 mg and 400 mg vials, each with a postreconstitution dilution of 25 mg/m.
Potential Side Effects:
The FDA-approved label for Empliciti (elotuzumab) lists common side effects including diarrhea, fatigue, fever, loss of appetite, constipation, cough, cold or flu symptoms, peripheral neuropathy (pain, tingling, or numbness in the extremities), nausea, and pneumonia.
Rare but critical side effects listed for Empliciti 300 mg/400 mg include increased risk for infection, increased risk for developing other cancers, infusion reactions, and liver damage.
References:
https://www.empliciti.com/
https://www.myeloma.org/empliciti-elotuzumab
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5013854/